 History: A 44 y.o. woman complaining of right flank pain was found to have a large retroperitoneal mass arising adjacent to the right adrenal gland (Fig. 1). At surgery, a 4,250 gram, 25.0 x 18.0 x 10.0 cm well-circumscribed multinodular mass with firm yellow gray-tan lobulated cut surfaces was removed (Fig. 2). A recurrence was detected ten months later and confirmed by FNA.
History: A 44 y.o. woman complaining of right flank pain was found to have a large retroperitoneal mass arising adjacent to the right adrenal gland (Fig. 1). At surgery, a 4,250 gram, 25.0 x 18.0 x 10.0 cm well-circumscribed multinodular mass with firm yellow gray-tan lobulated cut surfaces was removed (Fig. 2). A recurrence was detected ten months later and confirmed by FNA.
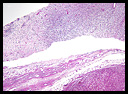
Microscopically, the tumor was composed predominantly of a hypercellular proliferation of atypical spindle cells, some with vacuolated cytoplasm. Much of the tumor had a myxoid appearance with scattered capillary-sized stellate blood vessels (Figs. 3, 4). Mitotic figures were variable, ranging from 0 to 5 per 10 hpf and occasional atypical forms were seen. Areas of transition from hypo to hypercellular regions were seen (Fig. 5). These areas exhibited variable histological patterns ranging from low grade to high grade myxofibrosarcoma and malignant fibrous histiocytoma (MFH) (Fig. 6). The tumor was positive for CD99 but negative for desmin and CD34. A Ki67 stain demonstrated considerable proliferative activity (Fig. 7)
Diagnosis: “Dedifferentiated Liposarcoma, Retroperitoneumâ€
Mingyi Chen MD, Donald R. Chase, MD
Department of Pathology, Loma Linda University and Medical Center
California Tumor Tissue Registry
Discussion: This case exhibits the typical histological features of dedifferentiated liposarcoma (DLS). Dedifferentiated liposarcoma is a malignant adipocytic neoplasm containing a non-lipogenic sarcomatous component of variable histological grade that arises against the background of a pre-existing “parent tumorâ€, usually a well-differentiated liposarcoma. DLS is one of four types of liposarcoma, generally classified by morphologic features and cytogenetic aberrations as:
(1) atypical lipomatous tumor/well-differentiated liposarcoma
(2) myxoid/round cell liposarcoma
(3) pleomorphic liposarcoma, and
(4) dedifferentiated liposarcoma.
The anatomical distribution of liposarcoma appears to be partly related to the histologic type. Atypical lipomatous tumor may occur in deep soft tissues of the limbs, but in the retroperitoneum, scrotum and mediastinum it is usually referred to as well-differentiated liposarcoma. Myxoid and/or round-cell liposarcomas and pleomorphic liposarcomas have a striking predilection for the limbs, and dedifferentiated liposarcoma occurs predominantly in the retroperitoneum.
The term dedifferentiated liposarcoma was first introduced by Evans in 1979, and refers to a well-differentiated liposarcoma or “atypical lipoma†that has histologically progressed into a higher-grade (less-differentiated) sarcoma, typically resembling MFH or fibrosarcoma. By definition, the pathologic diagnosis of dedifferentiated liposarcoma requires the concurrent identification of the 2 components, that is, well-differentiated liposarcoma and a cellular nonlipogenic sarcoma in the same lesion. Documented change in phenotype over time (recurrence) is also acceptable. Dedifferentiated liposarcoma usually occurs in late adult life, and most commonly in the retroperitoneum. Tumors in this area usually carry the worst prognosis. Most cases of dedifferentiated liposarcoma (90%) are found de novo, rather than in recurrence (10%) from well-differentiated lipomatous neoplasms. In 1997, Hericks et al studied 155 cases of dedifferentiated liposarcoma and found that neither the extent nor the grade of the dedifferentiated component of the tumor had a significant influence on the subsequent clinical behavior.
Amplification of chromosome 12q13-21 region has been associated with the development of liposarcomas. The most common chromosomal translocation is the FUS-CHOP fusion gene, which encodes a transcription factor necessary for adipocyte differentiation. Amplification of the MDM2 gene has been described in retroperitoneal dedifferentiated liposarcoma. The retinoblastoma protein (pRB) plays a major role in dedifferentiation and a ‘two-hit’ mechanism appears to be involved in altered retinoblastoma protein expression in dedifferentiated liposarcomas.
Liposarcoma is largely an H&E diagnosis, preferably made by excisional biopsy. Claim is made for diagnosis by FNA, however caution is urged. Lipocytic tumors are notorious for having multiple different phenotypes within the same tumor and FNA may not provide the more global survey necessary for diagnosis. Immunohistochemical evaluation is similarly of limited usefulness. Although tradition has taught that lipoblasts are necessary for the diagnosis, this is not the case, particularly in myxoid/round cell LS. When present, however, vacuolated cells with hyperchromatic nuclei indented by the vacuole are helpful. These lipoblasts have the ability to produce and accumulate non–membrane-bound lipid within the cytoplasm which indents the nucleus and causes a scalloping of the nuclear membrane. Lipid staining has generally fallen into disrepute, though older texts suggest Sudan black or oil red O stains. It must be emphasized, however that the diagnosis should be made by histomorphology, not special stains. Specifically, in our experience, S100 is not a helpful stain.
Multiple histological patterns may be present in dedifferentiated liposarcoma. About 5-10% cases of DLS may exhibit heterologus differentiation including other sarcomatous phenotypes of fibrosarcoma, dermatofibrosarcoma protuberans, hemangiopericytoma, rhabdomyosarcoma, leiomyosarcoma, chondrosarcoma, and osteosarcoma. The dedifferention may be high grade or low grade, and a soft tissue adage is that a fibrohistiocytic-appearing tumor in the retroperitoneum is a dedifferentiated LS until proven otherwise. Since the dedifferentiated component may mimic other sarcomas, immunostains may be important to exclude them.
DLS is a high-grade sarcoma that generally has a worse prognosis than its “parent tumor†with a local recurrence rate of 41%, a metastatic rate of 17%, and disease-related mortality of 28%. Complete surgical resection of the primary lesion at initial presentation is critical to achieving a cure or long-term survival.
Suggested Reading:
Enzinger FM, Weiss SW. Soft Tissue Tumors, 4th ed, CV Mosby, St Louis, MO, 2001.
Dei Tos AP, Pedeutour F. Dedifferentiated liposarcoma. In: Fletcher CDM, Unni KK, Mertens F, eds. Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press:38–39. World Health Organization Classification of Tumours; vol 5. 2002.
Evans HL. Liposarcoma: a study of 55 cases with reassessment of its classification. Am J Surg Pathol, 3:507–523, 1979.
Weiss SW, Rao VK. Well-differentiated liposarcoma (atypical lipoma) of deep soft tissue of the extremities, retroperitoneum, and miscellaneous sites: a follow-up study of 92 cases with analysis of the incidence of “dedifferentiation.†Am J Surg Pathol, 16:1051–1058, 1992.
Henricks WH, Chu YC, Goldblum JR, Weiss SW. Dedifferentiated liposarcoma: a clinicopathological analysis of 155 cases with a proposal for an expanded definition of dedifferentiation. Am J Surg Pathol, 21:271–281, 1997.
Elgar F, Goldblum JR. Well-differentiated liposarcoma of the retroperitoneum: a clinicopathologic analysis of 20 cases, with particular attention to the extent of low-grade dedifferentiation. Mod Pathol 1997;10:113–120.